About Opill
See why Opill may be the right choice for your patients.
Opill is available over the counter at retailers nationwide, online and via subscription.

A contraceptive option that fits your patient’s lifestyle
Opill is a progestin-only, estrogen-free contraceptive pill making it suitable for most* people of reproductive age, including those who12:
- Are breastfeeding
- Are smokers
- Have a history of migraines
*Do not use Opill® if you have or ever had breast cancer. See Opill® label for list of warnings.

Simple to use
Opill is dosed once daily at the same 0.075 mg norgestrel dose.3 For maximum contraceptive effect, Opill should be taken no later than three hours from the time the tablet was taken the day before.3
People who take Opill more than three hours late or miss one or more tablets should3:
- Take one tablet immediately, then resume Opill on their regular schedule
- Use a condom or other barrier method every time they have sex during the 48 hours after restarting Opill
- Take a pregnancy test or talk to their healthcare provider if their period is late after missing any tablets in the last month, or if they suspect they may be pregnant
How Opill works
Watch this video to learn more about Opill's mechanism of action and see why Opill can be an excellent birth control option for your patients.
Progestin-only pills like Opill work by:
- Increasing cervical mucus viscosity to inhibit sperm penetration3,10
- Suppressing ovulation in some cycles3,11
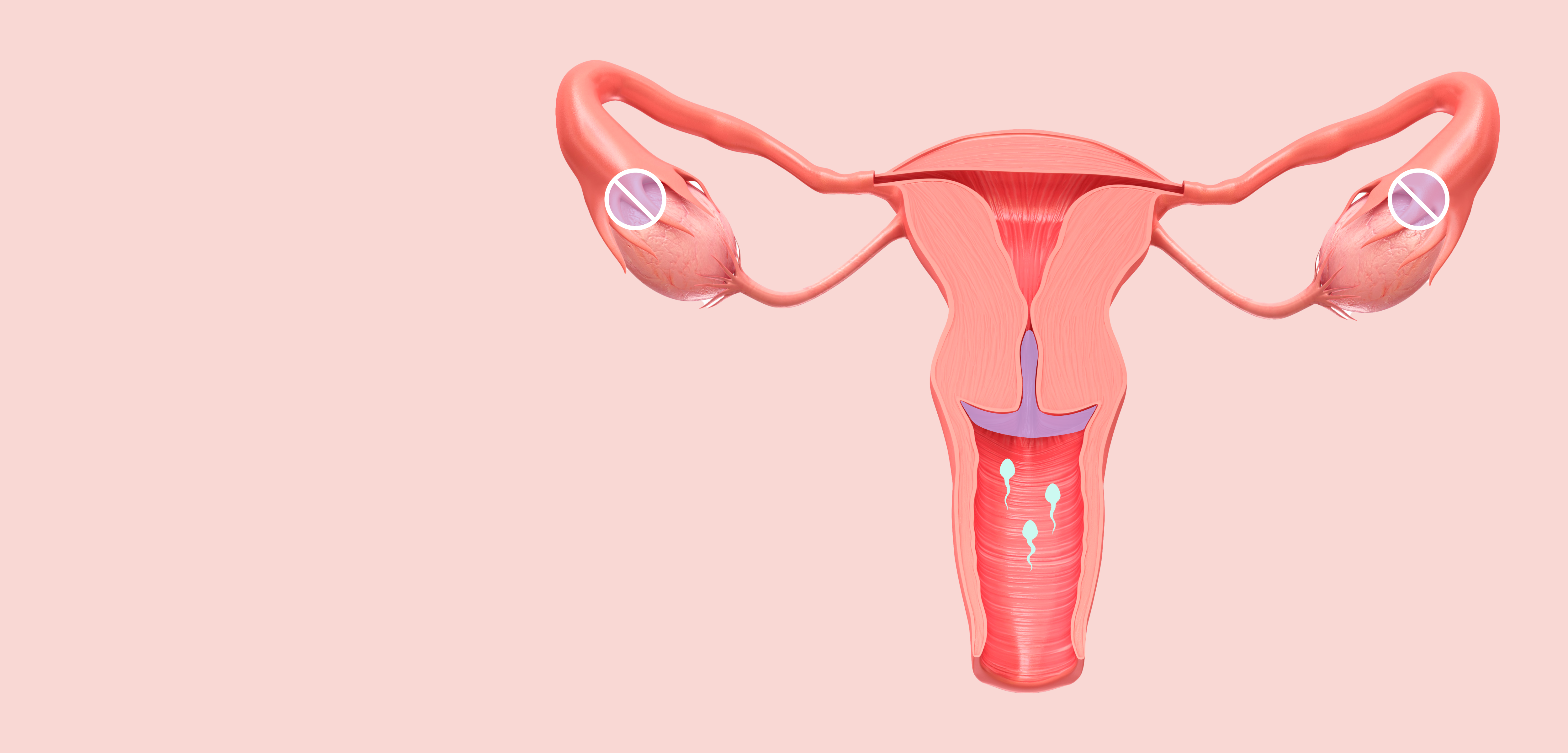
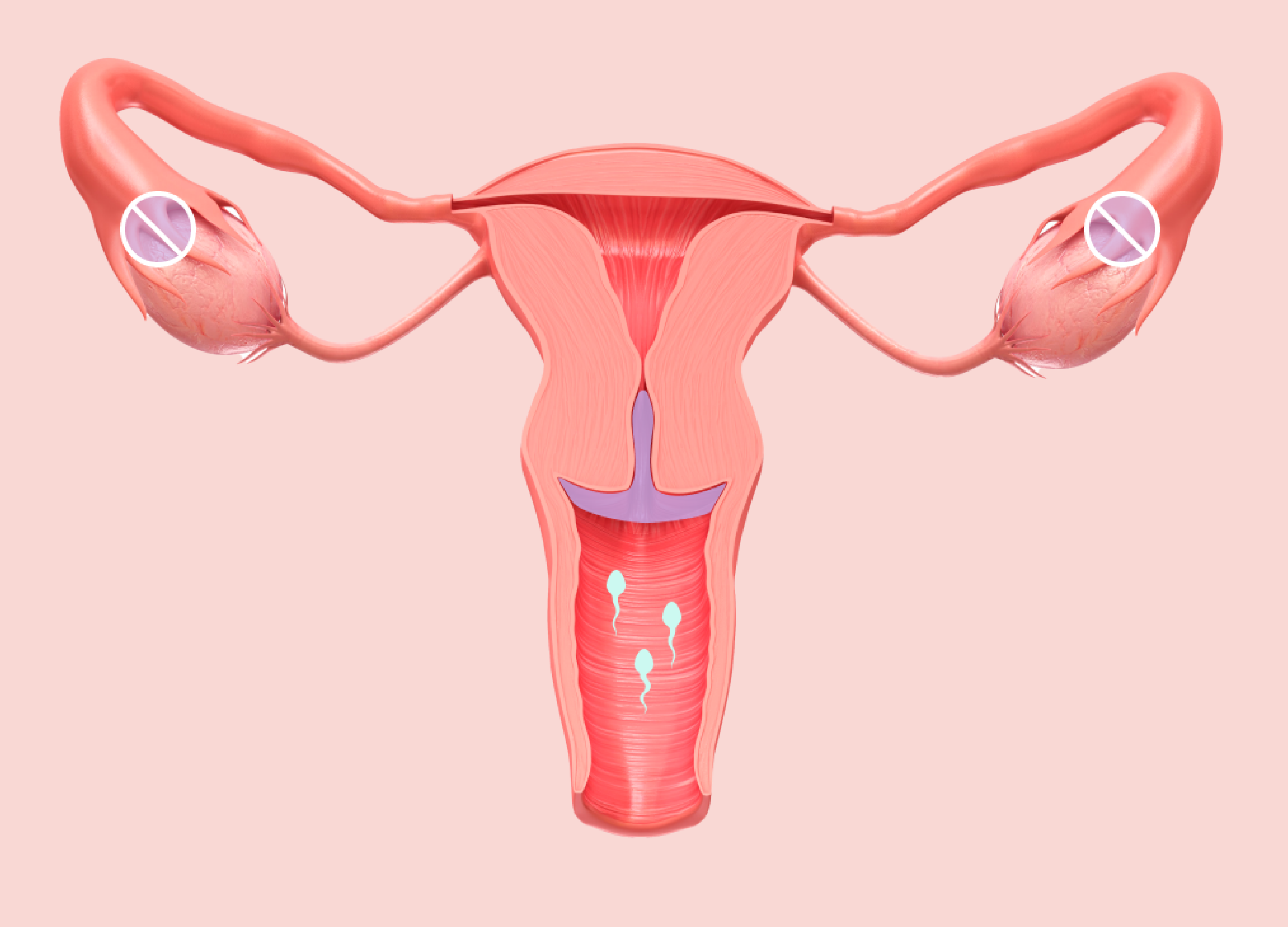
Suppresses or disrupts ovulation in some cycles
Increasing cervical mucus viscosity to inhibit sperm penetration
Opill efficacy
Opill is the most effective contraceptive available OTC.*1,2
It is 98% effective at preventing pregnancy when used as directed.3
Opill starts working 48 hours after initiation.*3
It can be initiated any day of the menstrual cycle — no need to wait for menses.3
Opill does NOT protect individuals from HIV/AIDS or other sexually transmitted diseases. It is NOT an emergency contraceptive and does not prevent pregnancy after unprotected sex.
*When used as directed.
Opill safety
A well-established safety profile supported by decades of real-world experience2
Side effects vary by individual, but Opill is generally well tolerated.13 The most frequently reported side effect is irregular bleeding.2,3
Ectopic pregnancy
Use of effective contraception decreases the risk of pregnancy and the risk of ectopic pregnancy overall.14 According to a recent US study, the risk of ectopic pregnancy with any oral contraceptive use is lower than with nonuse.14 Opill is not contraindicated for use in those with a history of ectopic pregnancy.12
No examinations or tests needed before initiating or continuing Opill4
Pelvic and breast examinations, cervical cancer screening and sexually transmitted infection screening are not required before initiating hormonal contraception.4,9 Studies have shown that individuals can use self-screening tools to determine their eligibility for hormonal contraceptive use.9 For further information, consult the US Medical Eligibility Criteria for Contraceptive Use and the US Selected Practice Recommendations for Contraceptive Use.
Who should not use Opill?
For further information about using progestin-only contraception in these situations, please refer to the CDC MEC.12
Opill should not be used by people who3:
- Have or ever had breast cancer
- Have allergies to this product or any of its ingredients, such as the color additive FD&C yellow No.5 (tartrazine)
- Are currently using another birth control pill, vaginal ring, patch, implant, injection or intrauterine device (IUD)
- Are pregnant or think they may be pregnant
- Are male
Consumers should talk to a physician before starting Opill if they3:
- Currently have vaginal bleeding between periods and have not already talked to a physician
- Have liver tumors or liver disease
- Have or ever had any cancer
Patient and consumer resources
Use these guides to prepare for discussions about Opill with your patients and customers.
